Biotech has always thrived on precision, but the pressure to generate fast, accurate insights has never been greater. Companies competing in therapeutics, diagnostics, and life-science tools face rising expectations from regulators, investors, and scientific partners. At the same time, research timelines are tightening. High-quality data must arrive earlier in development, with fewer errors and clearer interpretation. These converging pressures have pushed imaging technologies to the center of modern R&D strategy. The microscopes and visualization tools used even five years ago often can’t meet today’s demand for single-cell resolution, faster workflows, and deeper proteomic context. Here’s why next-gen technology matters.
Why High-Resolution Platforms Are Becoming Non-Negotiable in Modern R&D
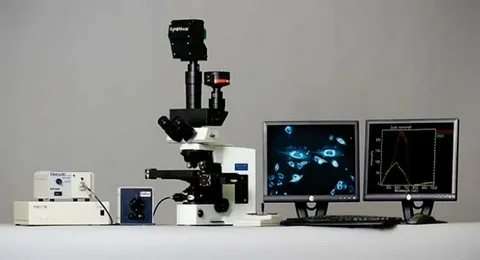
In the last decade, biological complexity has become more visible, and therefore more measurable. Traditional microscopy remains foundational, but many biotech teams now recognize its limitations when studying protein localization, cell-to-cell variability, or subtle molecular changes that influence therapeutic success. This shift is driving interest in advanced tools such as a protein microscope, which enables high-resolution proteomic visualization at scales that once required multiple instruments and much heavier workflows.
A protein microscope allows researchers to observe protein organization and interactions with a clarity that accelerates hypothesis generation and removes guesswork. This advantage becomes critical when developing therapies that depend on understanding intracellular behavior, mechanistic pathways, or target validation. With regulators expecting cleaner data earlier and R&D teams racing to eliminate dead-end candidates, next-gen imaging platforms offer a measurable competitive edge.
How AI-Driven Biotechnology Strengthens the Push Toward Precision
Precision doesn’t happen in isolation. Advances in AI and computational biology are accelerating the industry’s appetite for high-fidelity imaging data. AI tools are reshaping biotech development by improving pattern recognition, expediting research workflows, and enabling smarter prediction models across the life sciences. But AI is only as valuable as the quality of the data it receives. This is where next-generation imaging becomes indispensable.
High-resolution imagery feeds machine-learning models with cleaner, denser information that enhances both analysis and predictive strength. When AI can accurately identify subcellular structures, detect protein clustering, or measure subtle phenotypic changes, biotech teams can uncover insights that would never emerge from manual interpretation alone. This connection between imaging and AI creates a multiplier effect. Better imaging improves AI output. Better AI output refines experimental design. And refined experimental design accelerates the entire R&D pipeline.
Compression of R&D Timelines and the Demand for Faster Validation
The speed at which biotech organizations must deliver results has changed dramatically. Investors want earlier proof-of-concept. Regulators want stringent validation. Clinical partners expect robust preclinical support. And internal teams need reliable data to make critical go/no-go decisions. Traditional imaging workflows, with slower acquisition speeds and less quantitative rigor, often break under these expectations.
Next-gen imaging platforms streamline validation. When a protein microscope or other advanced system can reveal localization patterns, target engagement, or pathway disruptions in minutes rather than days, teams can run more experiments in less time. This acceleration enables iterative testing, where hypotheses are refined rapidly and ineffective candidates are eliminated early. Faster validation reduces the cost of failure, which is vital in a field where failure is inevitable but expensive. Biotech firms adopting advanced imaging platforms are not simply working faster, they are working smarter.
Competitive Positioning in a Market Built on Differentiation
Biotech companies often compete on differentiation including unique mechanisms of action, superior data packages, or innovations with stronger mechanistic insight. High-resolution imaging contributes directly to this narrative. When companies can visualize protein-level behavior with exceptional clarity, they tell a more compelling scientific story, one that investors, partners, and regulatory reviewers find persuasive.
This competitive edge extends beyond storytelling. Firms with more advanced imaging capabilities often uncover biological nuances that others miss, allowing them to file stronger intellectual property, refine therapeutic strategies, or identify first-in-class opportunities. Biotechs are increasingly judged by the sophistication of their tools, both in R&D and in the way they communicate scientific breakthroughs. Adopting next-gen imaging platforms signals that a company is serious about precision, data quality, and innovation at scale.
Workflow Modernization and the Need for Systems That Scale
Imaging is no longer a stand-alone task performed by a specialist in a dim lab. Modern R&D workflows require imaging systems that integrate with automation, data management platforms, and computational pipelines. Imaging tools must fit into multidisciplinary teams that include cell biologists, data scientists, chemists, and computational modelers. Older systems often become bottlenecks that are slow, incompatible with digital systems, or unable to handle the high-throughput demands of contemporary research.
Next-gen imaging platforms address these challenges by merging usability with scalability. They generate standardized data, reduce human variability, and connect seamlessly with downstream analytical tools. For growing biotech companies, this integration becomes essential. As teams expand or pivot, imaging systems must support new assays, different cell types, and emerging scientific needs without requiring months of retraining or reconfiguration.